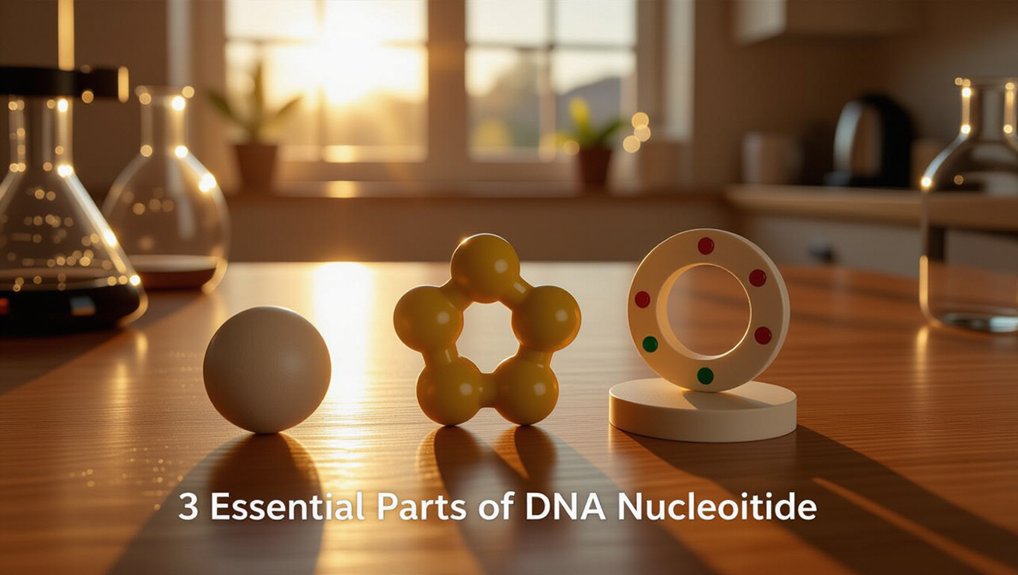
Every DNA nucleotide has three crucial parts that work together as a tiny team. The sugar, called deoxyribose, forms the main structure. The phosphate group attaches to the sugar and links one nucleotide to the next. The nitrogen base holds the actual genetic information like a coded message. Bases pair in specific ways which helps DNA copy itself. Together these three parts build the famous double helix and there is much more to investigate about them.
- Key Takeaways
- Introduction to Nucleotide Structure and Components
- Part One Nitrogenous Base Definition and Function
- Types of Nucleotide Bases Purines and Pyrimidines
- Part Two Five Carbon Sugar Ribose and Deoxyribose
- Sugar Role in Nucleotide Stability and Structure
- Part Three Phosphate Group Chemical Properties
- How Three Parts of Nucleotide Bond Together
- Nucleotide Structure in DNA Versus RNA Comparison
- Building Blocks Three Parts Form Nucleic Acids
- Phosphodiester Bonds Linking Three Part Nucleotides
- Function of Each Three Parts in Genetic Information
- Key Differences Between Three Nucleotide Components
- Study Guide Three Parts of Nucleotide Explained
- Frequently Asked Questions
Key Takeaways
- A DNA nucleotide has three essential parts: a phosphate group, a deoxyribose sugar, and a nitrogenous base.
- The phosphate group links nucleotides together, forming the negatively charged sugar-phosphate backbone of DNA.
- The deoxyribose sugar is a five-carbon sugar lacking an oxygen at the 2′ carbon, distinguishing DNA from RNA.
- The nitrogenous base (adenine, thymine, cytosine, or guanine) carries genetic information and participates in complementary base pairing.
- Together, these three components enable DNA’s structure, stability, and role in storing and transmitting genetic information.
Introduction to Nucleotide Structure and Components

At the heart of every cell, nucleotides act like tiny building blocks for DNA and RNA.
Each nucleotide has three main parts that always work together.
First is a sugar called a pentose sugar. In DNA it is deoxyribose.
In RNA it is ribose. This creates helpful nucleotide variations.
Next is a phosphate group. Phosphates link sugars together.
They form a strong sugar‑phosphate backbone that holds DNA and RNA.
Nucleotide importance also comes from energy.
Some nucleotides have one, two, or three phosphates.
Triphosphates like ATP store and carry energy for cell work.
Part One Nitrogenous Base Definition and Function
A nitrogenous base is the information center of a nucleotide. It holds genetic instructions. Each base works like a letter in a shared family code. Together they pass traits from one generation to the next. This shows deep nitrogenous base significance for every living community.
- Bases join in exact pairs and keep DNA strands close and stable.
- These base pairing mechanisms follow simple rules that students can learn and trust.
- The base sequence guides protein building so cells act in harmony.
Types of Nucleotide Bases Purines and Pyrimidines

Inside every DNA and RNA strand, nucleotide bases fall into two main groups called purines and pyrimidines. Purines include adenine and guanine. Their purine properties come from having two connected rings. Pyrimidines include cytosine, thymine, and uracil. Their single ring shape explains many pyrimidine roles.
In DNA, adenine pairs with thymine using two hydrogen bonds. Guanine pairs with cytosine using three hydrogen bonds. In RNA, adenine pairs with uracil instead of thymine, yet guanine still pairs with cytosine. When purines always pair with pyrimidines, the double helix stays stable and information stays reliable.
Part Two Five Carbon Sugar Ribose and Deoxyribose
After learning how bases pair up, it helps to look at what holds them.
That “holder” is a five carbon sugar. It can be ribose or deoxyribose.
Ribose structure includes an -OH group on the 2′ carbon. Deoxyribose has only hydrogen there.
- Ribose belongs to RNA nucleotides and links base and phosphate together.
- Deoxyribose function centers on building DNA nucleotides and keeping genetic messages safe.
- The sugar type decides if a nucleotide is a ribonucleotide or deoxyribonucleotide.
These small changes shape stability and reactivity for life’s shared genetic language.
Sugar Role in Nucleotide Stability and Structure

Sugar acts like the steady frame that keeps each nucleotide strong and in the right shape.
It forms the backbone of DNA with tight links between neighboring nucleotides.
This support is called sugar stability and it helps DNA stay safe over time.
Deoxyribose sugar lacks one oxygen atom at the 2′ carbon.
This missing piece makes DNA less reactive and harder to break down.
Ribose in RNA has that extra hydroxyl group so it breaks more easily.
Sugar configuration also sets the 5′ to 3′ direction.
This polarity guides copying and reading of genetic instructions.
Part Three Phosphate Group Chemical Properties

The phosphate group acts like the spark of energy and structure in each nucleotide. It holds a phosphorus atom joined to four oxygen atoms. Its negative charge helps DNA and RNA stay apart yet together in a shared watery space. This charge also supports phosphate stability in long chains.
- The phosphate group sits on the 5′ carbon of the sugar.
- Its negative charge helps DNA dissolve in water and stay protected.
- In ATP and other triphosphates, it fuels energy transfer and cell signals like cAMP.
How Three Parts of Nucleotide Bond Together

When looking at a nucleotide, it helps to picture three close teammates.
Each part holds a special place in the group. The base connects to the sugar through covalent interactions at the 1′ carbon. The phosphate joins the sugar at the 5′ carbon. These nucleotide bonding mechanisms keep the team firmly linked. Then one nucleotide reaches out to another. A phosphate forms a phosphodiester bond with the next sugar’s 3′ hydroxyl group. This creates a strong sugar phosphate backbone.
| Part | Joins To | Bond Type |
|---|---|---|
| Base | Sugar 1′ carbon | Covalent |
| Phosphate | Sugar 5′ carbon | Covalent |
| Nucleotide 1 | Nucleotide 2 sugar 3′ | Phosphodiester |
| Chain Start | Free 5′ phosphate | Directional |
| Chain End | Free 3′ hydroxyl | Directional |
Nucleotide Structure in DNA Versus RNA Comparison

Side by side, DNA and RNA look like close cousins with small but important differences.
In this nucleotide comparison, both carry sugar, phosphate, and a base.
DNA uses deoxyribose sugar. RNA structure uses ribose, which has one more oxygen.
That tiny change makes RNA bendy and less stable than DNA.
- DNA bases: A, T, C, G
- RNA bases: A, U, C, G
- Both use phosphodiester bonds for the backbone
DNA pairs A with T and G with C.
RNA pairs A with U and G with C.
These patterns guide how cells read and share instructions.
Building Blocks Three Parts Form Nucleic Acids

Picture a tiny construction kit that every cell uses to build DNA and RNA.
Each nucleotide has three main parts that always work together.
First is the nitrogenous base. It can be adenine, guanine, cytosine, thymine, or uracil. These bases create nucleotide variations that support genetic coding in every organism.
Second is the sugar. DNA uses deoxyribose and RNA uses ribose. This small sugar change matters.
Third is the phosphate group. It acts like a connector and gives the nucleotide a charged end.
Together, these three parts form strong, meaningful nucleic acids.
Phosphodiester Bonds Linking Three Part Nucleotides
Each three-part nucleotide becomes truly useful once it can link arms with its neighbors.
This joining happens through phosphodiester bond formation.
The 5′ phosphate of one nucleotide connects to the 3′ hydroxyl of the next sugar.
Together they build a strong sugar phosphate backbone that holds everyone in place.
- The bond forms by a condensation reaction that releases a molecule of water.
- Nucleotide chain directionality appears, with one 5′ end and one 3′ end.
- These bonds protect the sequence of bases and allow long DNA and RNA strands.
Function of Each Three Parts in Genetic Information

Although a DNA nucleotide looks simple, every one of its three parts has a special job.
The nitrogenous base holds the actual code. Its A-T and G-C pairing keeps messages clear and supports genetic stability. The order of these bases gives instructions for building proteins and shaping traits.
The sugar, deoxyribose, connects each base to the backbone. It helps the DNA strand keep its shape.
The phosphate group links sugars into a strong chain. This chain protects information during copying. When any part changes, especially the base, nucleotide mutations can appear and sometimes change an organism’s phenotype.
Key Differences Between Three Nucleotide Components

Even though all nucleotides share three main parts, those parts are very different. Each part has its own job and design. Together they create amazing nucleotide variations. The nitrogenous base can be a purine or pyrimidine. This choice guides base pairing. The sugar can be ribose or deoxyribose. One tiny -OH group marks the difference. The phosphate group links nucleotides into a strong backbone and can store energy. Triphosphate forms like ATP act as potent energy carriers that cells depend on.
- Nitrogenous base type
- Kind of pentose sugar
- Number of phosphate groups
Study Guide Three Parts of Nucleotide Explained

A quick study guide helps make the three parts of a nucleotide feel simple and clear.
First comes the nitrogenous base, like adenine, thymine, cytosine, or guanine.
Adenine pairs with thymine using two tiny hydrogen bonds, helping hold DNA together.
Next is the sugar called deoxyribose, which has one less oxygen than RNA sugar.
Then the phosphate group links sugars into a strong sugar-phosphate backbone chain.
These parts are built during nucleotide synthesis and changed during nucleotide metabolism.
Nucleotides may have one, two, or three phosphates, which affects energy and cell work.
Frequently Asked Questions
How Are Nucleotides Synthesized Inside Cells From Simpler Molecules?
Nucleotides are synthesized as cells weave nucleotide synthesis pathways, gradually shaping precursor molecules—ribose, bases, phosphates—into living code, inviting every molecule into a shared architecture where each completed nucleotide feels crucial, included, and part of a greater biological story.
What Diseases Result From Defects in Nucleotide Metabolism or Structure?
Defects in nucleotide metabolism or structure cause nucleotide disorders and metabolic deficiencies such as gout, Lesch–Nyhan syndrome, SCID, certain anemias, and cancers, often bringing fatigue, pain, immune problems, and shared experiences through lifelong medical support communities.
How Do Environmental Factors Like Radiation Damage Nucleotide Components?
Radiation effects break nucleotide bonds, alter bases, and create strand breaks, much like glitching a shared group playlist. Cells activate nucleotide repair pathways—excision, recombination, and proofreading—to restore DNA integrity, protecting the whole cellular “community” from mutation.
Can Diet or Supplements Influence Nucleotide Levels in Human Cells?
Yes, diet and supplements can influence cellular nucleotide levels. Through shared dietary sources like meats, fish, legumes, and fortified foods, plus thoughtfully used nucleotide supplements, communities support DNA synthesis, repair, and immune resilience together.
How Do Antiviral or Chemotherapy Drugs Target Specific Nucleotide Parts?
Antiviral or chemotherapy drugs, through almost mythic precision, use nucleotide targeting to mimic or block bases, sugars, or phosphates; these antiviral mechanisms hijack viral or cancer replication while largely sparing healthy cells, protecting the body’s shared cellular integrity.